Prozac, and other selective serotonin reuptake inhibitors (SSRIs), work by blocking the nerve cells from transporting serotonin back into the cell after it's been released. In a way, this is like stuffing a rag in the drain of a kitchen sink with the water running. If you block the transporter, the level of serotonin will rise, just like water in the sink, because it has nowhere to go. So, by administering an SSRI, a doctor can "block off the drain" and raise brain serotonin levels within several hours. The problem with this, as a lot of people are aware, is that it takes one or two months of SSRI treatment to see an antidepressant effect. This lag in the effect of SSRIs makes it pretty clear that depression is not an easy-to-fix "chemical imbalance."
So why is there such a gap in time between administration of SSRIs and response? To explain, a little background is needed.
In order to model depression in animal models, researchers use stress paradigms (paradigm in this sense just applies to the technique and its theoretical justification) to induce behavioral states that are considered analogs of human depression. Chronic stress, mostly through hormones called glucocorticoids, is known to significantly limit neurogenesis in the hippocampus of animals. Neurogenesis is a term that more or less encapsulates the birth, survival, growth, and movement of new nerve cells in adults. Researchers have shown that depression decreases neurogenesis by injecting a fluorescent marker known as BrdU into 'depressed' mice. BrdU is a chemical that attaches to cells that have recently split, or been 'born.' After subjecting mice to a stress paradigm, and observing depressed behavior, the brains can be checked for neurogenesis.
Normal neurogenesis looks something like this:
In depression, these cells do not make it through the survival phase.
Over the years, experimental evidence has shown that SSRIs do promote neurogenesis in a brain region called the hippocampus. Neurogenesis, while far from the only effect of SSRIs, was certainly present when mice improved in depressive symptoms. After observing this, many people wondered whether this were the elusive mechanism through which SSRIs have their antidepressant effect.
So was the neurogenesis a necessary mechanism or just a coincidence? In a 2003 study, researchers irradiated the hippocampus of mice that received antidepressants (this is similar to cancer treatments, lead shields are used to protect the adjacent areas) and succeeded in eliminating 87% of the new cells. With the loss of neurogenesis, the behavioral recovery that normally coincides with administering these drugs to depressed mice was absent.
This was reasonably conclusive evidence that neurogenesis was necessary for SSRIs to have their effect. In addition, the time course of antidepressant effect in clinical cases, four to eight weeks, is about the same time that it takes for new cells to be born and then integrate themselves into the neural network of the hippocampus in these rodent studies.
Seems straightforward right? When an animal is stressed, it releases glucocorticoids, which stop neurogenesis, and then the animal becomes depressed. Give it antidepressants, which kick start neurogenesis, and it goes back to normal. There would seem to be a negative correlation between neurogenesis and depression. In other words, neurogenesis goes up and depression goes down, or vice versa. Well, not so much.
A recently published a paper in the journal Biological Psychiatry shows evidence that neurogenesis is causal in relieving depression, but not in starting it.
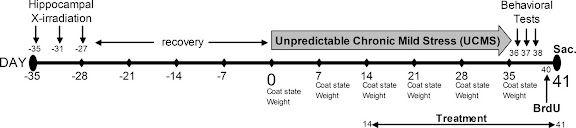
The researchers again used irradiation to suppress neurogenesis, and a paradigm known as unpredictable chronic mild stress (UCMS) to induce a depressed state. UCMS basically freaks out the mouse by repeatedly subjecting it to things like cage tilting, damp sawdust (lining the bottom of the cage), predator sounds, putting the mouse into another male's empty cage, and shifting the light/dark cycles.
They quantified the effects of this stress through assessment of the animal's coat state, weight, a splash test, the novelty-suppressed feeding (NSF) test and an actimiter. The coat state is an obvious sign that UCMS is working; a stressed mouse won't groom itself as much and it may start to fluctuate in weight. The other tests look for behavior analogous to depression in humans.
The paper actually has a nice graphic to illustrate the experimental design:
The paper contains about four experimental conditions, and I'll address them in the sequence that the authors did.
In the first set of conditions, the authors were simply looking to see if, when subjected to UCMS, there was a difference between mice who had their hippocampus irradiated and those that didn't. In other words, were mice without hippocampal neurogenesis more vulnerable to stress and would they show depressive signs quicker than normal mice?
In the above graph, and the ones that follow, a higher score indicates a worse coat condition. A low score can be interpreted as a "happier" mouse.
As you can see, there is virtually no difference in the behavioral effects of stress on the two groups of mice. The authors' interpretation of these results was that neurogenesis doesn't play a causal role in the pathogenesis of depression.
This is a significant finding because in the past it's been hard to dissociate neurogenesis and depression. Hippocampal neurogenesis is increased by nearly every antidepressant drug. Also, fluoxetine, imiprimine (a potent tricyclic antidepressant), and cannibinoid antidepressant therapies have been shown to require neurogenesis to have their effect.
So the question was, if neurogenesis isn't causing depressive symptoms, is it always needed to relieve them?
The authors decided to do a larger set of three experiments, where they would split the conditions appropriately. The only difference between the graphs below is the drug used in each. Unlike above, all of the mice discussed below were subject to the UCMS paradigm.
The first two graphs are basically a replication of the earlier finding that the monoaminergic drugs fluoxetine and imiprimine fail to relieve depression when neurogenesis is suppressed:As expected, only the groups that received the monoaminergic antidepressants with no irradiation were unaffected by the UCMS.
The next two graphs show the same thing, but with different drugs. The experimental drugs shown are SSR149415, a V1b receptor antagonist, and SSR125543, a CRF1
receptor antagonist (antagonists inhibit the function of a receptor). Both of these drugs work on the HPA axis, a neuroendocrine system that regulates a plethora of body functions. The HPA axis is usually overactive or somehow dysregulated in anxious or depressed humans, leading to an abnormally high level of glucocorticoids. This is not a good thing, seeing as glucocorticoids regulate neuronal survival, the size of structures like the hippocampus, the storage of new memories and the emotional appraisal of events. Too much of them, and you get things like hippocampal atrophy and a reduction in neuropil.
These two drugs inhibit processes in the HPA axis that are central to the release of glucocorticoids. To return to the kitchen sink simile, it's like turning down the faucet.
There are several novel drugs like SSR149415 and SSR125543 in clinical trials for anxiety and depression right now, and the authors wanted to see if they were also reliant on neurogenesis.
Here were the results:
Both groups responded to the treatment whether or not they were irradiated. So it seems that these drugs act on a different mechanism.
The authors suggest a model to explain their findings, whereby the hippocampal neurogenesis acts to repair the inhibition of the HPA axis.
T-shaped lines indicate a decrease, arrows an increase.
The general idea is that these new antidepressants potentially skip a step and directly influence the HPA axis.
This study is helpful in that it tells us something about the role of neurogenesis in depression. However, V1b and CRF1 receptor antagonists will not be game changers. It seems that they don't work any faster than current medications, and the irradiation factor is clinically irrelevant.
My personal opinion is that we won't make any real breakthroughs until we define the neural circuits involved in mood modulation and discover how the flow of information through them is affected by the disease.
All graphs and figures were adapted from Surget et al.
References:
- Surget et al. Biol Psychiatry. 2008 Aug 15:64(4):293-301. Epub 2008 Apr 11. dx.doi.org/10.1016/j.biopsych.2008.02.022
- Pariante CM and Lightman SL. Trends Neurosci. 2008 Jul 31. [Epub ahead of print] dx.doi.org/10.1016/j.tins.06006





11 comments:
Great post! You made a complicated topic quite understandable to someone without a science background, such as myself.
Looking forward to more.
If the neurogenesis hypothesis of resolving depression is correct, and the rat research translates to humans, intermittent fasting or calorie restriction may be of benefit to women but not men. Politically incorrect or not women are probably not well designed to function well psychologically if obese.
The full version of the abstract below is at http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=2405949&blobtype=pdf .
PLoS ONE. 2008 Jun 11;3(6):e2398.
Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males
Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W 3rd, Prabhu V, de Cabo R, Maudsley S, Mattson MP.
Laboratory of Neurosciences, National Institute on Aging Intramural Research Program, Baltimore, Maryland, United States of America. martinbro@grc.nia.nih.gov
The level of dietary energy intake influences metabolism, reproductive function, the development of age-related diseases, and even cognitive behavior. Because males and females typically play different roles in the acquisition and allocation of energy resources, we reasoned that dietary energy intake might differentially affect the brains of males and females at the molecular level. To test this hypothesis, we performed a gene array analysis of the hippocampus in male and female rats that had been maintained for 6 months on either ad libitum (control), 20% caloric restriction (CR), 40% CR, intermittent fasting (IF) or high fat/high glucose (HFG) diets. These diets resulted in expected changes in body weight, and circulating levels of glucose, insulin and leptin. However, the CR diets significantly increased the size of the hippocampus of females, but not males. Multiple genes were regulated coherently in response to energy restriction diets in females, but not in males. Functional physiological pathway analyses showed that the 20% CR diet down-regulated genes involved in glycolysis and mitochondrial ATP production in males, whereas these metabolic pathways were up-regulated in females. The 40% CR diet up-regulated genes involved in glycolysis, protein deacetylation, PGC-1alpha and mTor pathways in both sexes. IF down-regulated many genes in males including those involved in protein degradation and apoptosis, but up-regulated many genes in females including those involved in cellular energy metabolism, cell cycle regulation and protein deacetylation. Genes involved in energy metabolism, oxidative stress responses and cell death were affected by the HFG diet in both males and females. The gender-specific molecular genetic responses of hippocampal cells to variations in dietary energy intake identified in this study may mediate differential behavioral responses of males and females to differences in energy availability.
PMID: 18545695
Indeed, in a recent paper it was shown that suppression of adult neurogenesis leads to an increased HPA-axis stress response in mildly stressful situations. So the proposed circular model between HPA axis and hippocampal neurogenesis model seems to be correct.
Neuroreport. 2009 Apr 22;20(6):553-7.
Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response.
Schloesser RJ, Manji HK, Martinowich K.
Laboratory of Molecular Pathophysiology, Mood and Anxiety Disorders Program, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland 20892-3711, USA.
Abstract
Stress and glucocorticoids are among the strongest inhibitors of adult hippocampal neurogenesis. Despite the known role of the hippocampus in negative feedback regulation of the hypothalamo-pituitary-adrenal axis, whether the loss of hippocampal neurogenesis affects this inhibition has not been examined. Here we tested whether suppression of adult neurogenesis affected the hypothalamo-pituitary-adrenal axis response. Our results show that suppression of neurogenesis leads to a potentiated hypothalamo-pituitary-adrenal axis response after an exposure to a mild stressor. This study suggests that suppressed neurogenesis regulates the hypothalamo-pituitary-adrenal axis response.
PMID: 19322118 [PubMed - indexed for MEDLINE]PMCID: PMC2693911Free PMC Article
Thanks for this! I have an assignment on the serotonin theory of Depression and the question is presented in a very biased way. This is just what I was looking for. I'm of to look up the research.
I never thought that I was invincible to STD s or pregnancy or anything else parents warn their kids about. I just didn’t think about it. I was in a monogamous relationship and thought that I asked the right questions. We even talked about marriage Scary. During that time I was in college and donated blood on a regular basis in efforts to help others when I could. As you may know, each donation is tested. Well, on July 6th I had a meeting with a Red Cross representative and was told that I had HIV. “What went through your mind when you heard that "Rose" Good question reader! To be honest, I thought my life was over, that I would ever find love, get married, have children or anything normal. Lucky for me I have an amazing support system. My family supported me then as well. who helped me in search of cure through the media.there we saw a good testimony of sister 'Kate' about the good work of Dr Akhigbe natural herbal medicine cure.then I copied his email address and contacted him. A good herbalist doctor with a good heart, he is kind, loving and caring. He replied back to my message and told me what to do. It after a week the doctor sent me my herbal medicine and instructed me how to take it.Yes it worked very well, after drinking it I went to the hospital for another test and everything turned negative. What a wonderful testimony I can never forget in my life. Dr Akhigbe is a man who gave me a life to live happily forever so all I want you all to believe and know that cure of HIV is real and herbs is a powerful medicine and it works and heals. Dr Akhigbe also used his herbal medicine to cure diseases like: HERPES, DIABETES, SCABIES, HEPATITIS A/B, STROKE, CANCER, ALS, TUBERCULOSIS, ASTHMA, PENIS ENLARGEMENT, MALARIA, LUPUS, DEPRESSION, HIV/AIDS, EPILEPSY, BACTERIAL, DIARRHEA, HEART DISEASES, HIGH BLOOD PRESSURE, PARKINSON'S, ALZHEIMER, HUMAN PAPILLOMAVIRUS, BACTERIAL VAGINOSIS, SCHIZOPHRENIA, JOINT PAIN, STOMACH PAIN, CHROME DISEASES, CHLAMYDIA, THYROID, MAR BURG DISEASES, MENINGITIS, ARTHRITIS, BODY WEAK, SMALLPOX, DENGUE, FEVER, CURBS, CHANCRE, ETC. You are there and you find yourself in any of these situations, kindly contact Dr Akhigbe now to help you get rid of it. Here is his email address:
drrealakhigbe@gmail.com or you can write to him on whats app with his phone number: +2349010754824.
My appreciation is to share his testimony for the world to know the good work Dr Akhigbe has done for me and he will do the same for you.
It breaks my heart to think about what lab mice have to go through in order to get me my SSRI drugs. It may not seem like a big deal when it's euphemized on paper, but these long-term, inescapable stressors are awful for the mice. At least it's better than the infamous forced swim test. I really hope that someday I will be successful at quitting pharmaceutical drugs, so that I won't be participating in inflicting these kind of horrors on thinking, feeling creatures. Depression is awful, and I cry sometimes when I think about scientists stressing and hurting mice so that they can be miserable and hopeless, just like me. :'( After decades of taking psych drugs and reading about depression, I believe that depression is usually brought on by interpersonal factors (abuse, job loss, poverty, divorce, grief, debt, meaningless/stressful work) instead of a "brain disease", and so the best cure should interpersonal (love, support, family, sense of purpose, prosperity, changing our toxic system). However, once you use drugs to change the brain instead of changing your life to change your brain, now you are flooding your brain and other organ systems with neurotransmitters, and now you really do have a brain disorder. I wonder if they euthanize these mice in the studies or if they just force them to suffer "discontinuation symptoms" when the study is over, which at least for me, are many times worse than any depressive symptoms I had in the first place.
Post a Comment